Armored RNA -- New Technology for Protecting RNA
By Brittan L. Pasloske, Director of Research Operations, Ambion, Inc.
Introduction
Armored RNA Technology
Benefits of Using Armored RNA
Introduction (Back to Top)
In the last several years, diagnostic companies and clinical reference labs have been developing assays to monitor the concentration of human immunodeficiency virus (HIV) and hepatitis C virus (HCV) in the plasma of infected patients. Both viruses have RNA genomes, and the assays have been designed to measure the concentration of the viral RNA. For this reason, RNA controls and standards are key components of the assays.
Currently, these diagnostic assays rely on RNA synthesized by in vitro transcription to produce the appropriate controls and standards. However, RNA preparations are very susceptible to degradation by ribonucleases. Ribonucleases are ubiquitous in the environment, most notably being present in fingerprint grease, plasma, and other bodily fluids. Reagents used to manufacture the RNA must be absolutely ribonuclease-free, and all downstream handling of the RNA must be performed with ribonuclease-free pipet tips and tubes. Carefully controlled environmental conditions and highly skilled techniques are required to routinely handle RNA. Additionally, after synthesis, the RNA must be stored in an environment that minimizes the chemical hydrolysis of the phosphate backbone. The storage solution should contain a metal chelator, and the pH should be less than seven. The process of producing RNA that is stable during long term storage must be carefully designed and controlled and is not trivial.
In some viral assays, wild-type infectious virus from a patient or from tissue culture is used as a positive control. There are several major drawbacks to these types of controls. First, both HIV and HCV have high mutation rates, and therefore the sequence of the genomic RNA in a single lot is undefined and will be different from lot to lot. This heterogeneity is undesirable as a control. Second, an infectious control is a potential health hazard to the lab workers manufacturing it and using it in the assay. Third, laws and regulations governing the transport of infectious materials increase the cost of these controls.
A method that produces RNA controls and standards that are non-infectious, completely characterized in their sequences, cannot be degraded during their manufacture and storage, and perform just like in vitro transcribed RNA would be a significant improvement over the methods currently used for the production of RNA controls and standards.
Armored RNA Technology (Back to Top)
Armored RNA (US Patents 5677124, 5919625, and 5939262), a technology developed by scientists at Ambion and Cenetron Diagnostics, capitalizes on the mechanism employed by RNA phages to protect their genomes from degradation. RNA phages are essentially comprised of a single genomic RNA of 3 to 4 kilobase in length encapsidated by 180 coat proteins. A notable feature of these bacteriophage is that the genomic RNA assembled within the particles is very resistant to ribonuclease attack, presumably protected by the coat proteins. Based on the strategy used by the bacteriophage to protect its genomic RNA, a novel method for producing RNase resistant RNA was developed.
The original strategy was to produce recombinant, replicating RNA bacteriophage containing the RNA sequence of interest. This idea was rejected because of the known difficulties of maintaining heterologous RNA sequences within replicating RNA bacteriophage. As well, it was feared that the low fidelity phage polymerases would introduce errors into the packaged RNA, making it less useful as a standard.
Instead, a plasmid-driven packaging system was designed to produce Armored RNA (see figure). DNA encoding a bacteriophage coat protein and the RNA sequence of interest were cloned downstream of an inducible promoter. The recombinant plasmid is transformed into E. coli. Upon induction, RNA encoding the coat protein and the sequence of interest are transcribed from the recombinant plasmid. The fidelity of the E. coli RNA polymerase is much better than that of the bacteriophage polymerase. The coat protein is translated from the RNA. At a critical concentration, the coat protein binds specifically to the RNA, leading to the assembly of pseudoviral particles or Armored RNA.
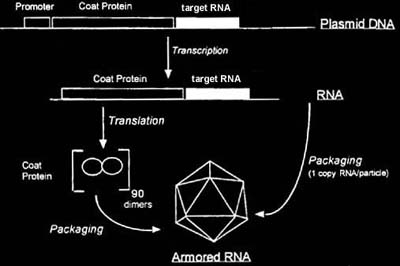
The resulting packaged RNA is completely resistant to ribonuclease digestion and is stable during long term storage. Once the packaged RNA is extracted from the protective coat protein, it is completely compatible with reverse transcription.
Benefits of Using Armored RNA (Back to Top)
- RNA sequences up to 1,000 bases can be packaged as an Armored RNA. Many different RNA sequences have been packaged: HIV, HCV, enterovirus, lambda, cyclophilin, GAPDH, 18S rRNA, and ten different human cytokines. All sequences tested have been successfully packaged.
- There is excellent lot-to-lot consistency. Different lots of the same Armored RNA construct, which are quantified by absorbance at 260 nm, produce identical signals under the same cycling conditions.
- The lot size of a single preparation will generate at least 1 x 1015 RNA copies. Since the material is stable, a single lot will last much longer than RNA made by in vitro transcription.
- Armored RNA may be added directly to total RNA and used directly as a quantitative standard. Incubation of Armored RNA at 70ºC for 5 minutes denatures the coat protein, thereby releasing the RNA and making it accessible to reverse transcriptase. This method precludes the need to extract the RNA from the Armored RNA. Competitive Quantitative RT-PCR kits (Ambion Inc.) are based on this application. Ten different Armored RNA cytokine standards were generated for performing quantitative RT-PCR to measure cytokine mRNA concentrations in total RNA.
- Armored RNA formulated with human plasma is stable at 4ºC for at least 11 months and at 37ºC for at least 30 days. This property makes Armored RNA ideal for producing positive controls for viral diagnostic assays.
- Armored RNA may be added directly to a plasma sample, making it useful as an internal quantitative standard.
- Armored RNA is stable at 45ºC for at least 5 days making it suitable for shipping at ambient temperature.
- If formulated in human plasma, Armored RNA is stable for at least 5 freeze-thaw cycles. Thus, these controls can be stored at –20ºC or –80ºC if preferred.
- Armored RNA is non-infectious to humans and therefore poses no potential hazard to lab personnel. This eliminates substantial additional charges for shipping infectious materials.
- DNA contamination of the Armored RNA is very low. The Armored RNA preparations demonstrate at least a 10,000-fold difference between the reverse transcriptase-plus and the reverse transcriptase-minus reactions.
Armored RNA technology is covered by U.S. patents 5,677,124, 5,919,625, and 5,939,262 and other patents are pending. Armored RNA is a registered trademark of Ambion and Cenetron Diagnostics.
For more information: Brittan Pasloske, Ambion Inc., 2130 Woodward St., #200, Austin, TX 78744-1832. Tel: 800-888-8804. Fax: 512-651-0201. Email: bpasloske@ambion.com.
