Bacterial Pili Structure Reveals Surprise
"These are the first detailed snapshots of the basis for an interaction between a disease-causing bacterium and its host," says Scott J. Hultgren, Ph.D., professor of molecular microbiology at Washington University School of Medicine. Hultgren, co-author on both papers, heads the research program that spawned the two imaging studies.
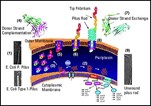
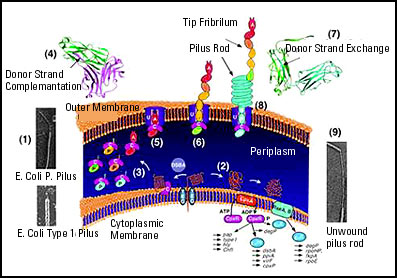
Over the past 10 years, Hultgren's group has determined how E. coli makes hair-like adhesive structure called pili. The pili enable bacteria to cling to tissue instead of getting swept away by bodily fluids such as urine. Without them, the bacteria cannot cause infections. Preventing pili from being made or functioning, therefore, could be an effective antimicrobial strategy.
Hultgren's team has identified the major players in pilus assembly. These include:
- protein subunits that eventually are assembled into pili,
- chaperones, boomerang-shaped proteins that ferry the subunits to the bacterial cell surface,
- doughnut-shaped proteins called ushers that assemble and extrude pili.
The first pilus subunits to emerge through the usher are different from the rest. These adhesins give the pilus the sticky tip that enables the bacterium to get a toehold in human tissue. Hultgren and colleagues previously showed that E. coli lacking adhesin are unable to infect the bladder. In collaboration with MedImmune Inc. (Gaithersburg, MD), they demonstrated in a mouse model that the adhesin can act as an effective vaccine by priming the immune system to disarm E. coli with the same protein. Hultgren also has been collaborating with a drug development company to develop therapeutics that will block the formation of bacterial pili and which therefore would be useful in the prevention and treatment of bacterial infections in humans.
Stefan D. Knight, Ph.D., associate professor of molecular biology, and postdoctoral fellow Devapriya Choudhury, Ph.D., at Uppsala Biomedical Center have obtained X-ray images of a chaperone (FimC) complexed to an adhesin (FimH). Hultgren's lab prepared the proteins from a strain of E. coli that infects the bladder. "The structure of the chaperone-adhesin complex tells us a story that is far more interesting than we ever could have imagined," Knight says. "Hypothetically, it could be the beginning of a new book on macromolecular assembly, protein folding and host-pathogen interactions."
FimH has two major regions, the images revealed. One interacts with chaperone, while the other -- which looks like a jelly roll -- latches onto a sugar called mannose. In the body, mannose is attached to a receptor in the bladder lining. The bacterium uses this receptor to gain access to the lining, where it can hide out. Knowing precisely how it interacts with mannose therefore should lead to ways of preventing this breaking and entering. "The molecular snapshot of our vaccine candidate also will provide new insights for our entire vaccine development program," Hultgren says.
At Washington University School of Medicine in St. Louis, Gabriel Waksman, Ph.D., associate professor of biochemistry and molecular biophysics, Klaus Fütterer, Ph.D., research associate, and Frederic G. Sauer, a graduate students, have obtained X-ray images of a different chaperone (PapD) complexed with an adaptor protein (PapK). The latter joins the adhesive tip to the pilus. The proteins were prepared in Hultgren's lab from a strain of E. coli that infects the kidney. "These images describe all of the interactions between the PapK subunit and the chaperone, and any of these interactions could be targeted for drug development," Waksman says. "If you perturb them, E. coli cannot form pili. That would prevent the bacterium from colonizing its host."
Both the PapK subunit and the part of FimH that interacts with the chaperone are barrel-shaped, made up of two curved sheets that resemble an immunoglobulin fold, a structure found in antibodies and many other proteins. However, unlike previously characterized immunoglobin folds, which are made up of seven strands (four in one sheet and three in the other), FimH, the adhesin, and PapK, the adaptor protein, lacked the seventh strand---the sheet that normally has three strands had only two.
The researchers discovered that each chaperone was an immunolgobulin fold that could temporarily share its seventh strand with a subunit. By taking the subunit under its wing, it created a complete barrel instead of one that was unstable without its seventh strand.
The researchers call the chaperone's action "donor strand complementation" -- the ability to lend the subunit one of its own strands without parting with that strand. In the living bacterium, donor strand complementation would stabilize the subunit during its journey to the cell surface, the researchers surmise. And they suggest that one strand of each subunit must complement a neighboring subunit in the completed pilus---a process they termed donar strand exchange. At the cell surface, each subunit would forsake its chaperone for another subunit, possibly with the usher's help.
"The crystal structures reported in this week's Science lift a corner of the great veil that has covered the action of molecular chaperones," comments David Eisenberg, D. Phil., in a perspective article that accompanies the two papers. Eisenberg, a professor of molecular biology at the University of California-Los Angeles, is a leading X-ray crystallographer of proteins.
The two papers also are relevant to research on diseases in addition to bladder and kidney infections such as middle-ear infections, pneumonia, meningitis, gonorrhea and many other infections that involve pili-making bacteria. "But what we're really excited about," Hultgren says, "is that these principles might apply to a wide range of biological fibers, such as the amyloid fibers that are important in Alzheimer's disease and the prion proteins associated with mad-cow disease and Creutzfeldt-Jakob syndrome. Therefore, we hope our findings will stimulate many new lines of research."
References:
Choudhury D, Thompson A, Stojanoff V, Langermann S, Pinkner J, Hultgren SJ, Knight SD. X-ray structure of the FimC-FimH chaperone-adhesin complex from uropathogenic E. coli. Science, Aug. 13, 1999.
This research was supported in part by the Swedish Research Council NFR, the Swedish Foundation for Strategic Research and the National Institutes of Health.
Sauer FG, Fütterer K, Pinkner JS, Dodson KW, Hultgren SJ, Waksman G. Structural basis of chaperone function and pilus biogenesis. Science, Aug. 13, 1999.
This research was supported in part by the National Institutes of Health.
Contact: Scott Hultgren, Department of Medical Microbiology, Washington University School of Medicine, 660 South Euclid Avenue, St. Louis, MO., 63110, 314-362-6772 (phone), 314-362-1998 (fax), or hultgren@borcim.wustl.edu (e-mail)
