HIV Drug Resistance Testing Improves Outcome for HIV Infected Patients
The NARVAL Study
Virco's Antivirogram
The NARVAL Study (Back to Top)
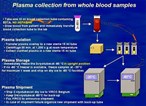
Having followed 541 patients for 24 weeks, the NARVAL study, conducted by l'Agence Nationale de Recherche sur le SIDA, is the largest prospective resistance testing study presented to date. Patients in the study consisted of heavily pre-treated patients who had received a median of seven prior drugs and had an average viral load of 4.3 log copies/ml and an average CD4+ count of 300 cells/ml. They were divided into three groups: standard of care group, who were treated without any resistance test information provided to the physician, and genotyping and phenotyping, both of which received testing of some kind, the results of which were used to assist the physician in the choice of treatment. The Visible Genetics' TrueGene HIV-1 Genotyping Kit was used in the genotyping group.
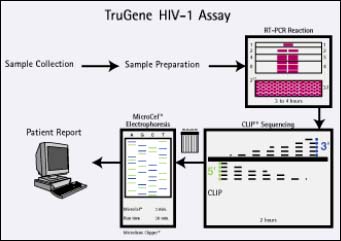
The percent of patients achieving viral suppression (less than 200 copies/ml), at 24 weeks, was greatest in patients in the genotyping group (36%), followed by those in the standard of care group (27%), compared with the phenotyping group (26%).
"We are pleased at the further confirmation that drug resistance testing using genotyping appears to be of benefit in the management of HIV patients undergoing antiretroviral therapy," said Richard Daly, CEO of Visible Genetics. "These data are consistent with results from previous studies and provide a compelling argument for the use of genotyping in HIV patient management.... Additionally, there were 19 other studies presented utilizing the TruGene HIV genotype test, showing the rapidly increasing acceptance of the company's important technology."
For more information: Visible Genetics, 700 Bay St., Ste. 1000, Toronto, ON, Canada, M5G 1Z6. Tel: 888-463-6844. Fax: 416-813-3250.
Virco's Antivirogram (Back to Top)
The Antivirogram is a diagnostic tool for monitoring phenotypic HIV-1 drug resistance, which combines in one assay system the phenotypic sensitivity testing of patient-derived HIV-1 strains against the different nucleoside reverse transcriptase inhibitors, non-nucleoside reverse transcriptase inhibitors, and protease inhibitors. Chimeric HIV-1 strains composed of sequences from the protease (PR), reverse transcriptase (RT), and segment of gag gene are isolated and amplified from the patient plasma viral RNA. These sequences undergo recombination inside CD4+ T-cells with a standard laboratory isogenic (HXB2) HIV-1 DNA construct from which the gag/PR/RT gene sequences has been deleted. From this a panel of recombinant HIV-1 strains is created, whose diversity reflects the population of virus sequences circulating in the patient.
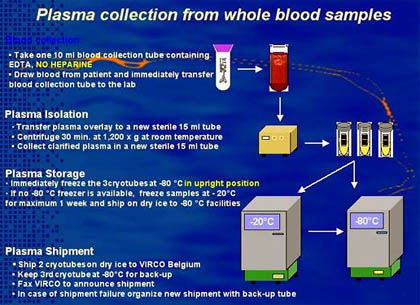
This study reported on enrolled 273 patients who had failed to respond to first-line therapy using a combination of drugs including at least two nucleoside analogue reverse transcriptase inhibitors and a protease inhibitor—currently the gold standard of HIV therapy. The patients were randomized to receive therapy based on phenotypic testing or on treatment history alone (standard of care).
Over 16 weeks, those using resistance testing experienced a significantly greater drop in viral load (-1.23 log vs. 0.87 log) and a greater proportion had a reduction in viral load to undetectable levels (59% vs. 42% <400 copies/ml). In addition, the number of active drugs—drugs to which the patients' virus was still sensitive—was significantly greater for those whose treatment was guided by resistance testing.
"In light of these and other supportive results, HIV drug resistance testing looks likely to become a standard element of routine clinical practice for people living with HIV/AIDS," commented lead investigator Cal Cohen, research director for the Community Research Initiative of New England.
The study was conducted in collaboration with 25 leading AIDS treatment centers and Glaxo Wellcome.
For more information: Virco n.v., Intercity Business Park, Generaal De Wittelaan L11 B4, 2800 Mechlen, Belgium. Tel: +32 15-28-63-37. Fax: +32 15-28-63-46.
