Human Monoclonals Induce Remyelination in Mouse Model
The researchers gave mice intracerebral injections of Theiler's murine encephalitis virus, which causes demyelination in the nervous system similar to the damage multiple sclerosis causes in humans. After the onset of demyelination, the mice were treated with two natural human monoclonal antibodies that bind to cells making myelin. This treatment promoted remyelination to an equivalent or greater extent than human intravenous immunoglobulin (IVIg), an established treatment for immune-mediated diseases.
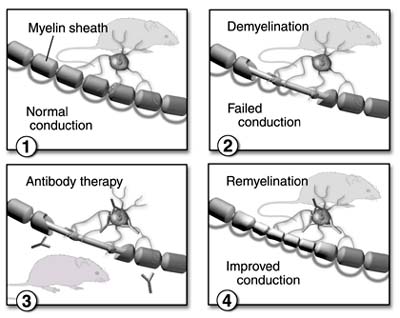
"Clearly, our study shows that the two natural human antibodies, when introduced into mice that had nervous system damage, safely caused substantial repair to the myelin and the nervous system," says Moses Rodriguez, a Mayo Clinic neurologist and the principal author of the study. "This is a significant step forward in our understanding of the nervous system and the immune system. Whereas we know that the immune system can have a protective effect on the body, we now are beginning to discover that the immune system may be harnessed to effect repair to the nervous system in the mouse model."
The mechanism mediating repair by these antibodies is unclear at present, and an understanding of the process is further confounded by the fact that not all surface-binding antibodies have this effect. However, the authors discuss two possible scenarios—a direct effect on myelin production by the interaction of the antibody with the cell surface, or an indirect effect, in which the presence of antibody on the surface signals the removal of damaged cells, allowing renewal to proceed.
Funding for the first stage of research was provided by the National Institutes of Health, the National Multiple Sclerosis Society, the Mayo Foundation, and Acorda Therapeutics. Acorda Therapeutics is a biotechnology company that develops therapeutic products for spinal cord injury and other central nervous system conditions. Acorda is planning to complete the preclinical work necessary before human clinical trials can be designed. This work includes scaling up manufacturing of the antibody to produce quantities sufficient for human clinical trials, and conducting formal toxicity and pharmacokinetic studies.
Ron Cohen, Acorda's president and CEO, states, "Acorda is proud of its collaboration with Dr. Rodriguez and Mayo Clinic. The identification of this human monoclonal antibody is a significant step forward in its development as a potential therapy for people with demyelinating conditions, such as multiple sclerosis."
For more information: Moses Rodriguez, Department of Neurology, Mayo Medical and Graduate Schools, Rochester, MN 55905. Email: rodriguez.moses@mayo.edu.
