Novel amplification technology to challenge PCR in diagnostics
By Tom Hollon
With PCR sitting pretty as king of the hill in nucleic acid amplification, who needs a competing technology? Well, the nucleic acids-based diagnostics market, for one: The diagnostics industry, in the view of Cytocell Ltd. of Banbury, UK, would welcome a challenger with a reliable and less expensive amplification technology.
And the time may be right for something new: "The market's still young in terms of nucleic acids-based diagnostics," says Don Cardy, Cytocell's research director. "There are plenty of immuno-based diagnostics, but not many based on nucleic acids."
Cytocell, a pioneer in fluorescence in situ hybridization (FISH) diagnostic tests, wants to take PCR on with novel isothermal (single temperature) amplification technologies it believes will be more economical for screening infectious bacteria and viruses.
One disadvantage of PCR, Cardy says, is the required investment in thermal cycling machinery. Cytocell's technologies amplify at a constant temperature, chosen in the range 37–41°C. "Because everything is done at one temperature," he says, "you could use a 96-well plate for the reactions and put it on a hotplate."
"With PCR," he goes on, "you have to change your annealing temperature and extension time to optimize for each individual target. You don't have to do that with ours—it's a standard reaction condition, whatever your target is."
How it works
Unlike PCR, which amplifies its target, Cytocell assays amplify a signal molecule—by transcribing a short strand of RNA—only if the target is present in the sample. Transcription of the signal RNA becomes possible only after the target nucleic acid, together with Cytocell's detection oligonucleotide probes, forms a unique structure called a three-way junction, or 3WJ. The 3WJ permits formation of a T7 RNA polymerase promoter essential for transcription.
The diagram illustrates a 3WJ for Cytocell's SMART assay (Signal Mediated Amplification of RNA Technology). Detection probes are designed to hybridize to the target and to each other, forming the 3WJ. The longer detection probe also has sequences for the T7 RNA polymerase promoter and downstream bases for signal transcription. After 3WJ formation, Bst DNA polymerase completes the second strand of the promoter by extending the shorter probe. T7 RNA polymerase can now bind its promoter and transcribe signal RNA. Signal molecules are in proportion to the target concentration, and can be measured in a variety of ways.
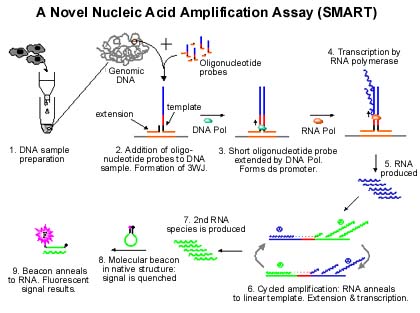
The beauty of the reaction, as Cardy points out, is that although the target-complementary probe sequences change from one target to the next, the detection signal (the vertical sequence being amplified) does not. "So you have standard reaction conditions every time."
Besides its use in detecting DNA and RNA from infectious organisms, SMART can detect allele variants in genetic diseases (three base deletions causing cystic fibrosis, for instance) and single nucleotide polymorphisms (SNPs). The company is developing another isothermal amplification assay that works without Bst, requiring only T7 RNA polymerase.
Versatile detection of amplified RNA is another advantage over PCR, according to Cardy. Detection assays can be colorimetric or fluorescent, in real-time, or after amplification is finished. "You can adapt our technology for any high throughput instrument that is around," he says. In its in-house assays, Cytocell uses ELOSA (Enzyme Linked Oligo-Sorbent Assays) and standard plate readers to detect the RNA signal. For real-time analysis, the company measures hybridization of signal molecules to molecular beacon oligo probes.
Cardy believes nucleic acid-based diagnostic tests for infectious organisms will prove more sensitive than immunological assays. That's what people in the market care about, he says. That, plus cost and simplicity. "We hope to produce a technology that is not expensive and that is simple to perform."
If the company's amplification techniques are simpler and cheaper, by Cardy's admission they still lag PCR in sensitivity. PCR can amplify on the order of 107 fold; SMART is currently amplifying on the order of 105. As indicated in the lower portion of the diagram, the original RNA signal can be boosted though an isothermal cycling amplification process, which Cardy believes will put SMART sensitivity on a par with PCR. The amplification cycling procedure is still in testing. If it works, Cardy believes Cytocell could have isothermal amplification products in the diagnostics market by 2002.
For more information: Don Cardy, Research Director, Cytocell Ltd., Somerville Court, Banbury Business Park, Adderbury, Banbury, Oxon OX17 3SN, UK. Tel: +44-1295-810910. Fax: +44-1295-812333. Email: d_cardy@cytocell.com.
About the author: Tom Hollon is a science writer and editor based in Rockville, MD. He was the founding editor of the journal Modern Drug Discovery. Prior to that, Tom conducted research at the National Institutes of Health, the Pasteur Institute, and the University of Washington. He can be reached at thollon@starpower.net.
