Phosphodiesterase inhibitors trigger apoptosis in cancerous colon cells
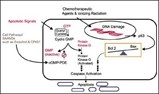
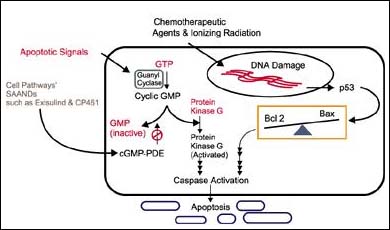
Cyclic nucleotide PDEs consist of 11 gene families, each having one or more isoforms. Each family of PDEs can bind and degrade cyclic AMP (cAMP) and/or cGMP, but differs in its immunological, physical and kinetic properties. Only a few PDE isoforms are expressed and used by any single type of cell or tissue to regulate cGMP or cAMP levels. Pharmaceutical developers have been studying these enzymes as potential drug targets to modulated cyclic nucleotide levels in diseases where their levels are important, such as asthma and heart disease. Until now, PDE inhibitors have not been developed as anti-cancer agents. Moreover, the majority of PDE inhibitors investigated to date do not induce apoptosis in tumor cells. Thus, exisulind and SAANDs represent a chemical class of unique PDE inhibitors.
"Exisulind and other SAANDs appear to act at an early point in the biochemical pathway that controls apoptosis and survival of cancer cells," said W. Joseph Thompson, vice president of research at Cell Pathways and lead author of the publication. "Our initial data indicates that increased PDE5 expression, and in some cases PDE2, occurs in precancerous and cancerous lesions. This over-expression appears to keep cGMP low in cells that have abnormal proliferation or apoptosis rates. The data indicate that by inhibiting cGMP PDE activity in cancer cells, a sustained rise in cGMP occurs, triggering cell death through protein kinase G. In normal cells, the cGMP pathway does not appear to be important in the regulation of apoptosis. Exisulind and other SAANDs restore the ability of abnormal cells to die by inhibiting that cGMP PDE activity."
Promising Clinical and Pre-Clinical Results
Cell Pathways has shown that Aptosyn and other SAANDs trigger apoptosis in abnormal cells in over 50 different tumor cell lines, as well as in animal models of various human cancers. Aptosyn has prevented or inhibited the growth of precancerous colon polyps in individuals with familial adenomatous polyposis (FAP), an inherited disease caused by a defect in the APC gene. Further, Aptosyn was found to reduce the rise in PSA levels in men with prostate cancer at risk of recurrence after prostatectomy. A New Drug Application (NDA) for Aptosyn is currently under review by the Food and Drug Administration (FDA) as a treatment for FAP.
Cell Pathways is conducting additional clinical trials with Aptosyn, both as a single agent and in combination with traditional chemotherapeutic agents against such cancers and precancerous conditions as prostate, breast and lung cancer, sporadic colon polyps (a precursor to colon cancer), and Barrett's esophagus (a precursor to esophageal cancer). Cell Pathways is also conducting Phase IB safety studies with a second SAAND compound, CPI 461, in cancer patients.
For more information: Cell Pathways, 702 Electronic Dr., Horsham, PA 19044. Tel: 215-706-3800. Fax: 215-706-3801.
